|
3.1 Vulval Cell Patterning
The cells that form the vulval toroids are the progeny of ventral hypodermal Pnp cells (EggFIG 7B) (Sulston and Horvitz, 1977). Twelve Pnp cells are born mid-L1. The six central cells P3p–P8p are endowed with equal potential to produce vulval cell lineages and are referred to as vulval precursor cells (VPCs) (EggFIG7A and EggFIG 8A). In L3, the VPCs are patterned so that vulval potential is restricted to the central three cells P5p–P7p. This patterning of the VPCs involves the combined action of three intercellular signaling events: an inductive signal emanating from the AC (LIN-3/LET-23 MAPK pathway activation), lateral signaling between VPCs (LIN-12/Notch), and signals from hyp 7 (reviewed in Greenwald, 1997; Sundaram, 2004; Sternberg, 2005).
As a consequence of patterning, P6p expresses a primary vulval cell fate and P5p and P7p secondary vulval fates. The remaining VPCs express a non-vulval tertiary fate, and their progeny fuse with the hypodermis (see Epithelial System - Hypodermis; Sternberg and Horvitz, 1986). Primary, secondary, and tertiary fates are recognized by the lineage pattern generated by a given VPC (EggFIG 7B and EggFIG 8A–C).
3.2 Vulval Morphogenesis
During the final round of vulval cell divisions, the primary descendants and some secondary descendants detach from the cuticle, allowing the vulval sheet to bend inward and the cells within it to rearrange their cell–cell contacts (EggFIG 8C). This invagination step establishes the beginnings of the vulval lumen, which continues to expand during morphogenesis. Proteoglycans and their associated glycosaminoglycans, likely expressed in vulval cells, are necessary for this step, although their precise role is not known (Herman and Horvitz, 1999; Bulik et al., 2000; Hwang et al., 2003). As morphogenesis continues, cells migrate toward the center of the developing vulval primordium and wrap around to meet their anterior/posterior homologs on the other side (EggFIG 9) (Sharma-Kishore et al., 1999). Homotypic cell fusions occur between cells of homologous fate, resulting in the formation of toroid or half-toroid rings (see Inoue et al., 2002 for a useful guide to vulval cell nuclei positions during and after morphogenesis).
As part of the process of joining vulval and uterine lumens, the AC creates a hole in the apex of the developing vulva (EggFIG 8A–C). In L3, while Pnp cells are dividing, the ventral hypodermal basal lamina and gonadal basal lamina break down precisely at the site of contact with the AC. The basolateral portion of the AC crosses through this gap, attaches to, then inserts between the descendants of the primary-fated P6p lineage cells. This invasion is stimulated by a diffusible signal from the primary cells (Sherwood and Sternberg, 2003). Later, P6p terminal progeny fuse, forming a toroid (vulF) around the invading AC process. The AC is then removed by heterotypic fusion with the utse, leaving a channel in the apex of the vulva (Newman et al., 1996). When the utse membrane is ruptured by passage of the first egg, uterine and vulval lumens become continuous.
During late L4, the vulval muscles attach to the vulval epithelial tube and to the body wall (see below). The tube then partially everts (turns inside out), generating the adult vulva in which the lumen is closed until vulval muscles contract (EggFIG 10) (Sulston and Horvitz, 1977; Sharma-Kishore et al., 1999).

4 Uterine and Vulval Muscles
The uterine (um1L/R, um2L/R) and vulval (vm1L/R, vm2L/R) muscles (EggFIG 1 and EggFIG 11A), collectively referred to as the sex muscles, are required for moving eggs through the uterus and vulva. Only 4 the 16 sex muscles receive direct inputs from the egg-laying neurons. The remaining sex muscles are electrically coupled, either directly or indirectly, to these innervated muscles (see EggFIG 13). This configuration may serve to coordinate uterine and vulval contraction. The sex muscles are classified as nonstriated muscles because they do not have the striated appearance (typified by body wall muscle) normally attributed to the presence of an ordered array of multiple sarcomeres (muscle contractile units; see Muscle System - Somatic Muscle). Vulval muscles have a single sarcomere that extends along the entire muscle length and attaches to a discrete zone in the body wall at one end and to the vulva at the other end (White, 1988). The uterine muscle myofilament network seems to be anchored to a thin basal lamina on the surface facing the uterus. In contrast to the vulval muscles, the attachment points are randomly arrayed and this distribution of dense bodies is similar to that seen in vertebrate smooth muscles (see Muscle System - Nonstriated).
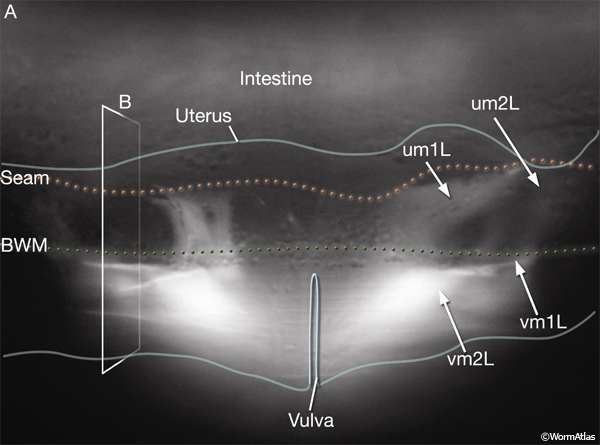 Eight uterine muscles are arranged in four bands around the uterus lobes: two bands per lobe, two muscle cells per band (EggFIG 1). A left/right pair of um2-type muscles (um2L/R) encircles the more distal ut toroids of each lobe. A left/right pair of um1-type muscles (um1L/R) cup the ventral half of the uterus over the more proximal ut toroids, and at their dorsal edges, they attach to the lateral seam. The ventral-proximal edges of the um2 muscles overlap with the um1 muscles (EggFIG 1). Uterine muscles are covered in a thin basal lamina (EggFIG 11C). The muscle filaments are circumferentially oriented so their contraction potentially moves eggs by squeezing on the uterus (EggFIG 11C) (Sulston and Horvitz, 1977). The uterine muscles are not directly innervated and are instead coupled via gap junctions, either directly or indirectly, to vulval muscles that are innervated by the egg-laying neurons (see EggFIG 13) (White et al., 1986).
Eight vulval muscles are organized around the vulva into two layers of four cells: One layer contains four vm1-type muscles and the other four contain vm2-type muscles. The muscles run at diagonal angles from the vulval lips to the subventral body wall (EggFIG 12A). Their proximal ends are linked to the vulval cuticle (for details, see Muscle System - Nonstriated).
EggFIG 12: The vulval muscles. A. DIC/epifluorescent image of an adult egl-15::GFP transgenic animal showing reporter expression in the eight vulval muscles, ventral view. Magnification, 1000x. (Strain source: C. Branda and M. Stern.) B. TEM, transverse section, of a late-L4 larva from the region indicated by the line in A. The image shows the ends of vulval muscles that interdigitate between the vulval toroids. (VNCL, VNCR) Ventral nerve cord, left and right fascicle, respectively; (BWM) body wall muscle. (Image source: L4 Vul [MRC] 4866-19.)
EggFIG 13: Connectivity of the vulval and uterine muscles. (um) Uterine muscle; (vm) vulval muscle; VC1-6, HSN, VA7, VB6, and VD7 are motor neurons. (Adapted, with permission, from White et al., 1986.)
The vm2 muscles attach between the uterus and vulF (EggFIG 12B). Their distal ends insinuate between adjacent members of the ventral body wall muscle quadrant (EggFIG 11B). vm1 muscles attach to the vulva more ventrally than do the vm2s, between vulC and vulD toroids (EggFIG 12B), but they join the body wall more dorsally, attaching near the dorsal edge of the ventral body wall muscle quadrant (EggFIG 11B).
The vm2s are the only sex muscles that are directly innervated (EggFIG 13) (White et al., 1986). The muscles extend arms into the regions of neuropil formed at the vulva where they receive synaptic inputs (see EggFIG 15A,B). vm1 connects to vm2 by gap junctions. Coordinated foreshortening of the vulval muscles pulls the lips apart, allowing eggs to pass through the lumen and out into the environment.
Uterine and vulval muscles derive from a common precursor, the sex myoblast (SM). During L1, the mesoderm (M) blast cell (EggFIG 14A,B) lineage produces a left and a right SM (SML/R) (Sulston and Horvitz, 1977). In L2, SML/R migrate anteriorly along ventral muscle quadrants to the precise center of the developing gonad and future vulva (EggFIG14C). There, the SMs undergo three rounds of division to produce the vulval and uterine muscle cells (EggFIG 14D) (for details on muscle specification programs, see Harfe et al., 1998; Corsi et al., 2000; Lui and Fire, 2000; Kosta and Fire, 2001; Eimer et al., 2002).
EggFIG 14A: Sex muscle development. Schematic showing the temporal order of events leading to establishment of the adult egg-laying system, lateral view, left side. The scale on the left indicates larval stage and corresponding hours (hr) post-hatching at 20°C. Figures showing DIC/epifluorescent images of corresponding stages are indicated in parentheses on the right. For cells of the M/SM and Pnp lineages, cell nuclei only are shown. (GP) Gonadal primordium; (VPCs) vulval precursor cells. *M is located on right side of the animal. **Of the egg-laying neurons (VC1-6 and HSNL/R), only HSNL/R axons extend into the nerve ring in the adult. (Based on Sulston and Horvitz, 1977; Li and Chalfie, 1990; Garriga et al., 1993.) (Strain source: B.D. Harfe, M. Krause and A. Fire.)
EggFIG 14B-D: Sex muscle development. DIC/epifluorescent (B,D) or epifluorescent only (C) images of hlh-8::GFP transgenic animals, lateral view, left side, at different stages of sex muscle development. (Colored dotted lines) Relative positions of the gonadal primordium or the developing vulva. Magnification, 1000x. (Strain source: B.D. Harfe, M. Krause and A. Fire.)
EggFIG 15: Egg-laying neurons. A. Epifluorescent image of an adult transgenic hermaphrodite expressing an ida1::GFP reporter gene in the egg-laying neurons VC1-6 and HSNL/R, ventral view. A few other neurons (not labeled) that have processes in the ventral nerve cord (VNC) also express this marker. Magnification, 400x. (Strain source: T. Zahn and J. Hutton.) B. Epifluorescent image of an ida-1::GFP transgenic adult in the region indicated by the box in A. Magnification, 1000x.
SM migration and positioning at the gonad center is guided by the balance of several forces: a gonad-dependent attractive (GDA) mechanism (Thomas et al., 1990), a gonad-dependent repulsive (GDR) mechanism (Stern and Horvitz, 1991), and a gonad-independent attractive (GIA) mechanism (Chen et al., 1997; Huang et al., 2003). The DUs, VUs, and AC of the SPh (EggFIG 14A) and primary-fated P6p vulval cells (EggFIG 7A) express FGF-related ligand EGL-17, which is likely to correspond to the GDA signal for SM migration. Interestingly, these same cells also appear to be the source of the GDR mechanism (Burdine et al., 1997, 1998; Branda and Stern, 2000).
5 Egg-laying Neurons
The vm2 muscles receive major inputs from two groups of motor neurons, the VCn neurons (VC1–6) and the HSNs (HSNL/R) (EggFIG 15A) (see White et al., 1986 and Neuron system for detailed descriptions of each neuron). The precise role of each neuron and the neurotransmitters they release in egg-laying appears to be complex; several models have been proposed (Weinschenker et al., 1995; Waggoner et al., 1998, 2000; Bany et al., 2003; Shyn et al., 2003).
VC4 and VC5 cell bodies flank the vulval epithelial tube and have short processes in the VNC. VC1, VC2, VC3, and VC6 neuron cell bodies are spaced along the length of the VNC. Each sends out a single main axon that runs in the dorsal “neighborhood” of the cord and makes similar synaptic contacts to one another. When VCn neuron axons reach the vicinity of the vulva, they send processes dorsally along the ventral basal (outer) surface of vulE (EggFIG 16). The neurons branch and synapse with one another and with the HSNs and vm2 muscle arms, forming a local neuropil (EggFIG 15B). VC4 and VC5 branch more extensively than other VC neurons in this region.
EggFIG 16A: Egg-laying neurons. Schematic of the adult hermaphrodite midbody region, dorsal view, showing the vulva, vulval muscles (vm), and egg-laying neurons (VC1 and VC2 cell bodies not shown). (BWM) Body wall muscle; (VNCL, VNCR) ventral nerve cord, left and right fascicle, respectively.
EggFIG 16B&C: Egg-laying neurons. TEMs, transverse sections, of an adult hermaphrodite at the axial positions indicated by the lines in A. (B Image source: N2U VC [MRC] 2178-24; C image source: N2U VC [MRC] 2179-22.)
The VCn neurons are derived from the anterior daughters of ventral hypodermal blast cells P3–P8, the same cells that produce VPCs (described above; see Sulston and Horvitz, 1977). The VCn neurons are born in L1, begin to send out processes in late L3, and branch in the region of the vulva during L4 (EggFIG 14A). Surprisingly, VCn branching depends on cells of the vulva and not on the presence of their targets (Li and Chalfie, 1990; Colavita and Tessier-Lavigne, 2003).
HSNL/R cell bodies are situated subventrally, just posterior to the vulva (EggFIG 15B). Each HSN axon projects ventrally to the midline to join the ipsilateral VNC (VNCR or VNCL) and from there extends into the nerve ring. As they pass the vulva, the HSNs defasciculate dorsally, branch, and form synapses with VCn neurons and vm2 muscle arms, thereby contributing to the neuropil.
HSNL/R are born in the tail of the embryo and migrate before hatching to the midbody, near the gonadal primordium (EggFIG 14A) (Sulston and Horvitz, 1977; Sulston et al., 1983; Desai et al., 1988). Axon outgrowth begins during L2 and L3 and is guided by primary-fated P6p lineage cells (described above) and VNC neurons (Thomas et al., 1990; Garriga et al., 1993). Synapse formation takes place in the L3 and L4 stages. Primary vulval cells (from which vulE is formed) produce a signal that acts as a synaptic guidepost, promoting the correct pattern of presynaptic vesicle clustering in the HSNs (Shen and Bargmann, 2003; Shen et al., 2004).
6 List of Cells in the Uterus
1. Late L2/early L3 stage SPh cells that give rise to the uterus
DU Z1.pap (Dorsal Uterine precursor; generates uterus, spermatheca and spermatheca-uterine valve cells)
VUs and AC are of either the 5R or 5L configuration:
5R configuration
VU, Z1.ppa (Ventral Uterine precursor; generates uterus, spermatheca and spermatheca-uterine valve cells)
AC, Z1.ppp (Anchor Cell)
VU, Z4.aaa (Ventral Uterine precursor; generates uterus, spermatheca and spermatheca-uterine valve cells)
VU, Z4.aap (Ventral Uterine precursor; generates uterus, spermatheca and spermatheca-uterine valve cells)
5L configuration
VU, Z1.ppa (Ventral Uterine precursor; generates uterus, spermatheca and spermatheca-uterine valve cells)
VU, Z1.ppp (Ventral Uterine precursor; generates uterus, spermatheca and spermatheca-uterine valve cells)
AC, Z4.aaa (Anchor Cell)
VU, Z4.aap (Ventral Uterine precursor; generates uterus, spermatheca and spermatheca-uterine valve cells)
DU, Z4.apa (Dorsal Uterine precursor; generates uterus, spermatheca and spermatheca-uterine valve cells)
7 References
Alkema, M.J., Hunter-Ensor, M., Ringstad, N. and Horvitz, H.R. 2005. Tyramine functions independently of octopamine in the Caenorhabditis elegans nervous system. Neuron 46: 247-250. Article
Bany, I.A., Dong, M.Q. and Koelle, M.R. 2003. Genetic and cellular basis for acetylcholine inhibition of Caenorhabditis elegans egg-laying behavior. J. Neurosci. 23: 8060-8069. Article
Branda, C.S. and Stern, M.J. 2000. Mechanisms controlling sex myoblast migration in Caenorhabditis elegans hermaphrodites. Dev. Biol. 226: 137-151. Article
Bulik, D.A., Wei, G., Toyoda, H., Kinoshita-Toyoda, A., Waldrip, W.R., Esko, J.D., Robbins, P.W. and Selleck, S.B. 2000. sqv-3, -7, and -8, a set of genes affecting morphogenesis in Caenorhabditis elegans, encode enzymes required for glycosaminoglycan biosynthesis. Proc. Natl. Acad. Sci. 97: 10838-10843. Article
Burdine, R.D., Chen, E.B., Kwok, S.F. and Stern, M.J. 1997. egl-17 encodes an invertebrate fibroblast growth factor family member required specifically for sex myoblast migration in Caenorhabditis elegans. Proc. Natl. Acad. Sci. 94: 2433-2437. Article
Burdine, R.D., Branda, C.S. and Stern, M.J. 1998. EGL-17(FGF) expression coordinates the attraction of the migrating sex myoblasts with vulval induction in C. elegans. Development 125: 1083-1093. Article
Ceol, C.J. and Horvitz, H.R. 2004. A new class of C. elegans synMuv genes implicates a Tip60/NuA4-like HAT complex as a negative regulator of Ras signaling. Dev. Cell 6: 563-576. Article
Chang, C., Newman, A.P. and Sternberg, P.W. 1999. Reciprocal EGF signaling back to the uterus from the induced C. elegans vulva coordinates morphogenesis of epithelia. Curr. Biol. 9: 237-246. Article
Chen, E.B., Branda, C.S. and Stern, M.J. 1997. Genetic enhancers of sem-5 define components of the gonad-independent guidance mechanism controlling sex myoblast migration in Caenorhabditis elegans hermaphrodites. Dev. Biol. 182: 88-100. Article
Colavita, A. and Tessier-Lavigne, M. 2003. A Neurexin-related protein, BAM-2, terminates axonal branches in C. elegans. Science 302: 293-296. Abstract
Corsi, A.K., Kostas, S.A., Fire, A. and Krause, M. 2000. Caenorhabditis elegans twist plays an essential role in non-striated muscle development. Development 127: 2041-2051. Article
Desai, C., Garriga, G., McIntire, S.L. and Horvitz, H.R. 1988. A genetic pathway for the development of the Caenorhabditis elegans HSN motor neurons. Nature 336: 638-646. Abstract
Eimer, S., Donhauser, R. and Baumeister, R. 2002. The Caenorhabditis elegans presenilin sel-12 is required for mesodermal patterning and muscle function. Dev. Biol. 251: 178-192. Article
Eisenmann, D.M., Maloof, J.N., Simske, J.S., Kenyon, C. and Kim, S.K. 1998. The beta-catenin homolog BAR-1 and LET-60 Ras coordinately regulate the Hox gene lin-39 during Caenorhabditis elegans vulval development. Development 125: 3667-3680. Article
Garriga, G., Desai, C. and Horvitz, H.R. 1993. Cell interactions control the direction of outgrowth, branching and fasciculation of the HSN axons of Caenorhabditis elegans Development 117: 1071-1087. Article
Greenwald, I.S., Sternberg, P.W. and Horvitz, H.R. 1983. The lin-12 locus specifies cell fates in Caenorhabditis elegans. Cell 34: 435-444. Abstract
Greenwald, I. 1997. Development of the Vulva. In C. elegans II (ed. D. L. Riddle et al.). chap. 19. pp. 519-541. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York. Article
Hanna-Rose, W. and Han, M. 2000. Getting signals crossed in C. elegans. Curr. Opin. Genet. Dev. 10: 523-528. Abstract
Harfe, B.D., Branda, C.S., Krause, M., Stern, M.J. and Fire, A. 1998. MyoD and the specification of muscle and non-muscle fates during postembryonic development of the C. elegans mesoderm. Development 125: 2479-2488. Article
Herman, T. and Horvitz, H.R. 1999. Three proteins involved in Caenorhabditis elegans vulval invagination are similar to components of a glycosylation pathway. Proc. Natl. Acad. Sci. 96: 974-979. Article
Huang, X., Huang, P., Robinson, M.K., Stern, M.J. and Jin, Y. 2003. UNC-71, a disintegrin and metalloprotease (ADAM) protein, regulates motor axon guidance and sex myoblast migration in C. elegans. Development 130: 3147-3161. Article
Hwang, H.Y., Olson, S.K., Esko, J.D. and Horvitz, H.R. 2003. Caenorhabditis elegans early embryogenesis and vulval morphogenesis require chondroitin biosynthesis. Nature 423: 439-443. Abstract
Inoue, T., Sherwood, D.R., Aspock, G., Butler, J.A., Gupta, B.P., Kirouac, M., Wang, M., Lee, P.Y., Kramer, J.M., Hope, I., Burglin, T.R. and Sternberg, P.W. 2002. Gene expression markers for Caenorhabditis elegans vulval cells. Mech. Dev. 119 Suppl 1: S203-209. Article
Karp, X. and Greenwald, I. 2003. Post-transcriptional regulation of the E/Daughterless ortholog HLH-2, negative feedback, and birth order bias during the AC/VU decision in C. elegans. Genes Dev. 17: 3100-3111. Article
Kim, S.K. 1997. Polarized signaling: basolateral receptor localization in epithelial cells by PDZ-containing proteins. Curr. Opin. Cell Biol. 6: 853-9. Abstract
Kimble, J. 1981. Alterations in cell lineage following laser ablation of cells in the somatic gonad of Caenorhabditis elegans. Dev. Biol. 87: 286-300. Abstract
Kimble, J. and Hirsh, D. 1979. The postembryonic cell lineages of the hermaphrodite and male gonads in Caenorhabditis elegans. Dev. Biol. 70: 396-417. Article
Kostas, S.A. and Fire, A. 2002. The T-box factor MLS-1 acts as a molecular switch during specification of nonstriated muscle in C. elegans. Genes Dev. 16: 257-269. Article
Levitan, D. and Greenwald, I. 1998. Effects of SEL-12 presenilin on LIN-12 localization and function in Caenorhabditis elegans. Development 125: 3599-3606. Article
Li, C. and Chalfie, M. 1990. Organogenesis in C. elegans: Positioning of neurons and muscles in the egg-laying system. Neuron 4: 681-695. Abstract
Liu, J. and Fire, A. 2000. Overlapping roles of two Hox genes and the exd ortholog ceh-20 in diversification of the C. elegans postembryonic mesoderm. Development 127: 5179-5190. Article
Newman, A.P. and Sternberg, P.W. 1996. Coordinated morphogenesis of epithelia during development of the Caenorhabditis elegans uterine-vulval connection. Proc. Natl. Acad. Sci. 93: 9329-9333. Article
Newman, A.P., White J.G. and Sternberg, P.W. 1996. Morphogenesis of the C. elegans hermaphrodite uterus. Development 122: 3617-3626. Article
Newman, A.P., White, J.G. and Sternberg, P.W. 1995. The Caenorhabditis elegans lin-12 gene mediates induction of ventral uterine specialization by the anchor cell. Development 121: 263-271. Article
Schedl, T. 1997. Developmental Genetics of the Germ Line. In C. elegans II (ed. D. L. Riddle et al.). chap. 10. pp. 417-500. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York. Article
Seydoux, G. and Greenwald, I. 1989. Cell autonomy of lin-12 function in a cell fate decision in C. elegans. Cell 57: 1237-1245. Article
Sharma-Kishore, R., White, J.G, Southgate, E. and Podbilewicz, B. 1999. Formation of the vulva in Caenorhabditis elegans: a paradigm for organogenesis. Development 126: 691-699. Article
Shemer, G. and Podbilewicz, B. 2003. The story of cell fusion: big lessons from little worms. Bioessays 25: 672-682. Abstract
Shen, K. and Bargmann, C.I. 2003. The immunoglobulin superfamily protein SYG-1 determines the location of specific synapses in C. elegans. Cell 112: 619-630. Article
Shen, K., Fetter, R.D. and Bargmann, C.I. 2004. Synaptic specificity is generated by the synaptic guidepost protein SYG-2 and its receptor, SYG-1. Cell 116: 869-881. Article
Sherwood, D.R. and Sternberg, P.W. 2003. Anchor cell invasion into the vulval epithelium in C. elegans. Dev. Cell 5: 21-31. Article
Shyn, S.I., Kerr, R. and Schafer, W.R. 2003. Serotonin and Go modulate functional states of neurons and muscles controlling C. elegans egg-laying behavior. Curr. Biol.13: 1910-1915. Article
Stern, M.J. and Horvitz, H.R. 1991. A normally attractive cell interaction is repulsive in two C. elegans mesodermal cell migration mutants. Development 113: 797-803. Article
Sternberg, P.W. and Horvitz, H.R. 1986. Pattern formation during vulval development in C. elegans. Cell 44: 761-72. Abstract
Sulston, J. E. and Horvitz, H. R. 1977. Post-embryonic cell lineages of the nematode Caenorhabditis elegans. Dev. Biol. 56: 110-156. Article
Sulston, J.E., Schierenberg, E., White, J.G., and Thomson, J.N. 1983.The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 100: 64-119. Article
Sundaram, M.V. 2004. Vulval development: the battle between Ras and Notch. Curr. Biol. 14: R311-313. Article
Thomas, J.H., Stern, M.J. and Horvitz, H.R. 1990. Cell interactions coordinate the development of the C. elegans egg-laying system. Cell 62: 1041-1052. Abstract
Vogel, B.E. and Hedgecock, E.M. 2001. Hemicentin, a conserved extracellular member of the immunoglobulin superfamily, organizes epithelial and other cell attachments into oriented line-shaped junctions. Development 128: 883-894. Article
Waggoner, L.E., Zhou, G.T., Schafer, R.W. and Schafer, W.R. 1998. Control of alternative behavioral states by serotonin in Caenorhabditis elegans. Neuron 21: 203-214. Article
Waggoner, L.E., Hardaker, L.A., Golik, S. and Schafer, W.R. 2000. Effect of a neuropeptide gene on behavioral states in Caenorhabditis elegans egg-laying. Genetics 154: 1181-1192. Article
Weinshenker, D., Garriga, G. and Thomas, J.H. 1995. Genetic and pharmacological analysis of neurotransmitters controlling egg laying in C. elegans. J. Neurosci. 15: 6975-6985. Article
White, J. 1988. The Anatomy. In The nematode C. elegans (ed. W. B. Wood). chapter 4. pp 81-122. Cold Spring Harbor Laboratory Press, New York. Abstract
White J.G., Southgate, E., Thomson, J.N., and Brenner, S. 1986. The structure of the nervous system of the nematode C. elegans. Philos. Trans. R. Soc. Lond. Ser. B. Biol. Sci. 314: 1-340. Article
|
